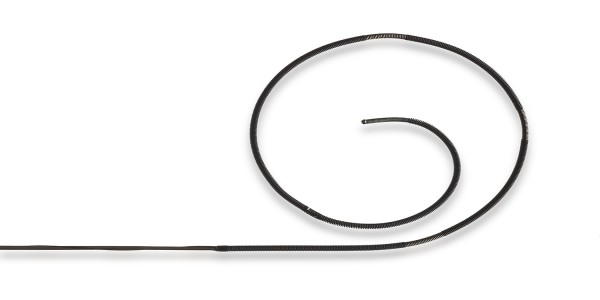
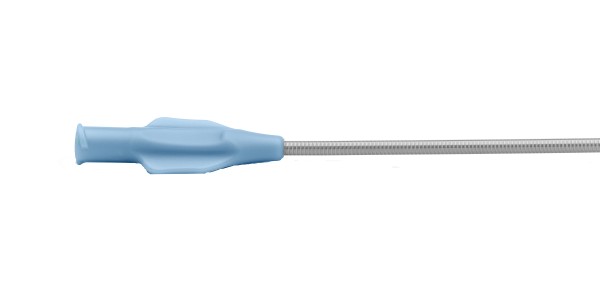
Finished Devices and Market-Ready Products
Get products to market faster by utilizing our selection of market-ready guidewires and catheter platforms that are regulatory-approved. Partner with us for access to expertise in regulatory, development, and manufacturing excellence.
Expand your capacity and simplify your supply chain with our fully integrated solutions and capabilities across the full spectrum of design, product/process development and documentation, regulatory affairs, and high volume/low-cost manufacturing.
With the experience of 13 US FDA 510(k) clearances and several product lines with EU CE Mark, we facilitate getting your device to market quickly and more efficiently.
Our team understands the challenges that you face. A strong medical advisory group is maintained to supplement clinical expertise and provide feedback on everything from product performance to long-term trends in clinical practice.
Leverage our full range of in-house product performance testing for support in the design and development process and compilation of testing required to secure regulatory clearances and approvals.