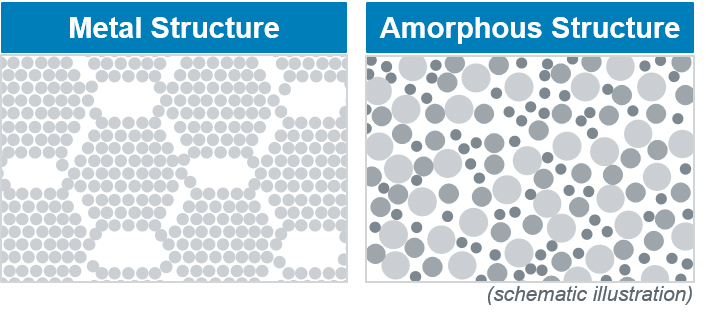
Amorphous metals are formed by the shock freezing of metallic melts. The atoms have no opportunity to form a crystalline lattice and solidify in a disordered manner (amorphous). Since the phase transformation from liquid to solid is suppressed in this process, no crystallization nuclei are formed during solidification. These defects in the lattice structure of conventional metals influence the mechanical and electromagnetic properties and lead, for example, to the material showing an increased tendency to corrosion, being brittle or cracking more quickly. By using amorphous metals, these risks can be avoided.
Amorphous Metals
Amorphous metals, also known as amorphous alloys or bulk metallic glasses, are undercooled frozen metallic liquids. They show material properties which normally exclude each other, i.e. high hardness and strength with high elasticity at the same time.
What makes Amorphous Metals so unique?
Since amorphous metals do not have lattice structures, no grain and phase boundaries are formed.
The material shows excellent mechanical properties, has above average corrosion resistance, is biocompatible and behaves isotropic.
For this reason, metallic glasses, as amorphous metals are also called, are excellently suited for a variety of high-tech applications and are interesting, for example, for wear-resistant drive components, stable suspensions, diaphragms for sensor technology or housings for consumer electronics.
What Properties do Amorphous Metals have?
Mechanical Properties of Amorphous Metals
Amorphous metals offer properties in a unique combination not found in conventional materials.
- High strength combined with excellent elasticity (twice higher than steel)
- High surface quality
- High hardness and low abrasion (comparable to ceramic)
- Isotropic properties
- Low electrical conductivity
Due to these properties, amorphous metals can be used well for components that are subject to high stress, such as hinges or gear parts.
Amorphous Metals show High Magnetic Permeability
- Electrical resistance is almost temperature independent
- Easy to magnetize and demagnetize
These properties make amorphous metals ideal for use in electric motors and EMI shielding applications.
Chemical and Medical Properties
In the chemical and medical environment, amorphous metals are characterized by
- High corrosion resistance due to the absence of grain boundaries and crystalline structures
- Biocompatibility of some alloys (ISO 10993-5 and ISO 10993-12 certified)
Due to these properties, amorphous metals can be used excellently for medical instruments or implants but also for components in corrosive environments.