Due to the significant stresses encountered in vivo, the strength of a bone cement is one of the most important factors for guaranteeing long-term fixation in total knee arthroplasty (TKA) and total hip arthroplasty (THA).3 After implantation, patient movement exposes the cement mantle to a complex blend of compressive loading combined with bending, impact, tension, torsion and shear.
The International Organization for Standardization (ISO) has specified procedures to test bone cements for each of these strengths in isolation. In 2014, Klaus-Dieter Kuehn published novel comparative data on the strength of several brands of bone cements when tested according to ISO 5833. For these tests, Kuehn used nine total samples of each cement (3 samples each from 3 different batches).
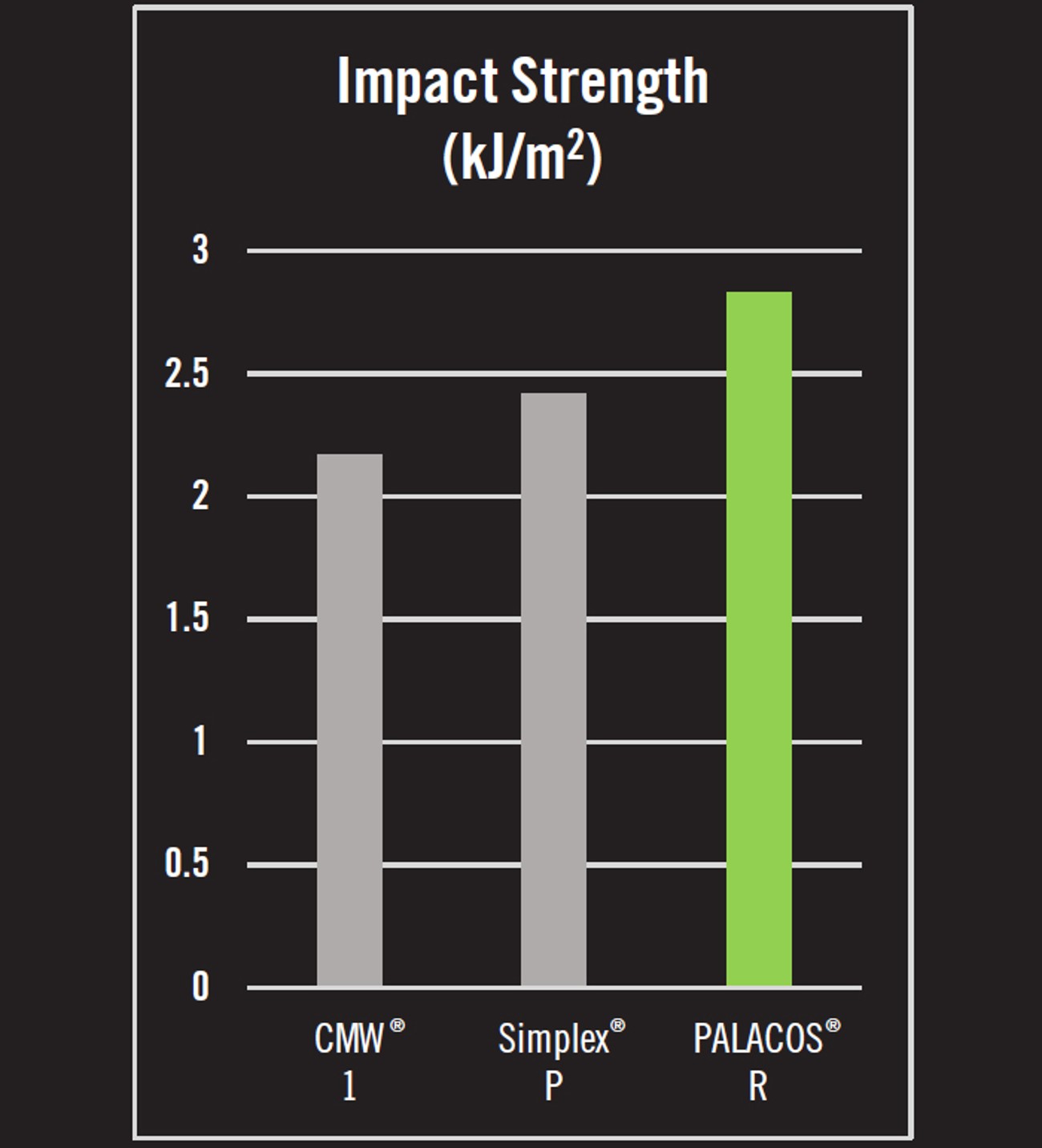
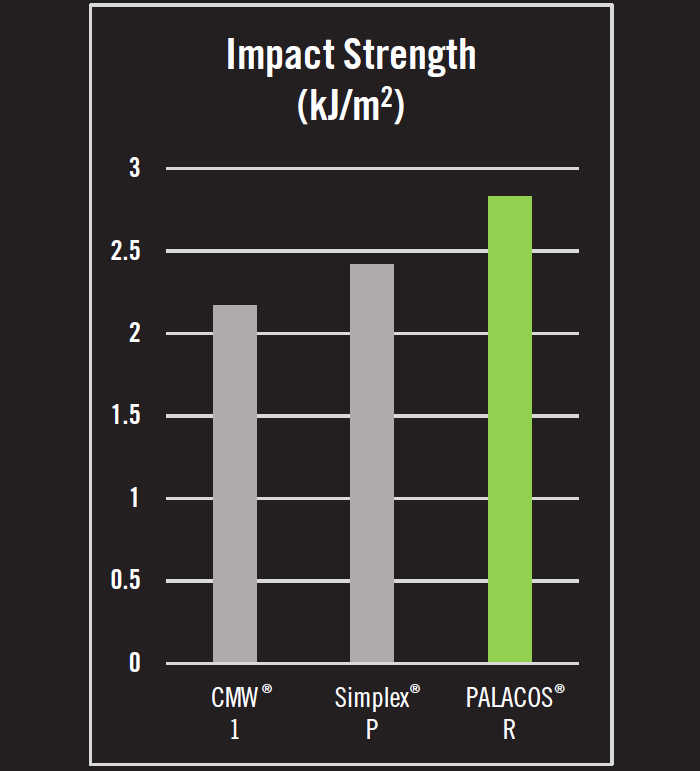
- Impact strength is the energy required to cause a fracture in the bone cement when struck by a sudden blow. In tests of impact strength, PALACOS® R possessed 16.9% and 30.4% greater impact strength than Simplex® P and CMW® 1, respectively.4
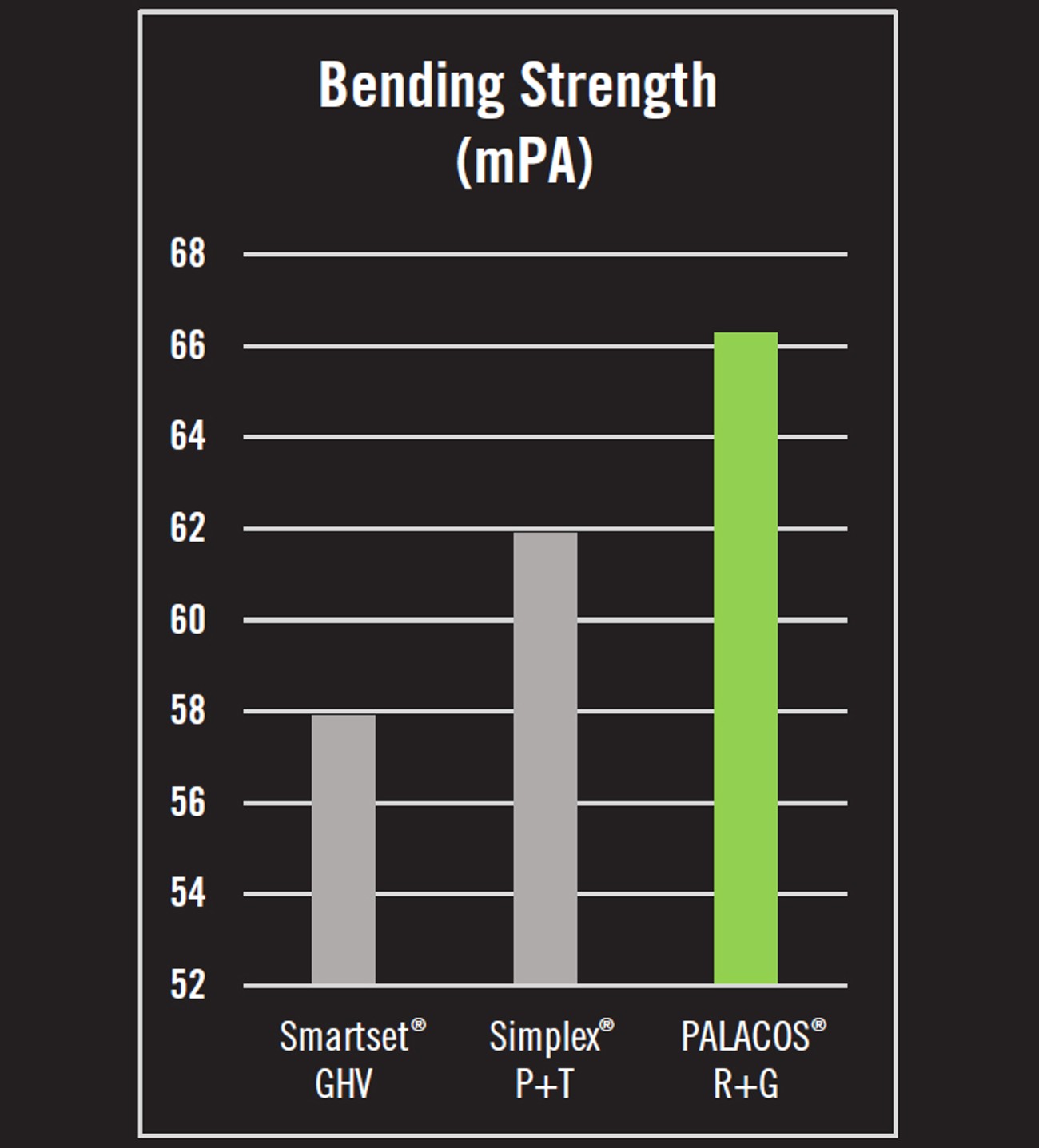
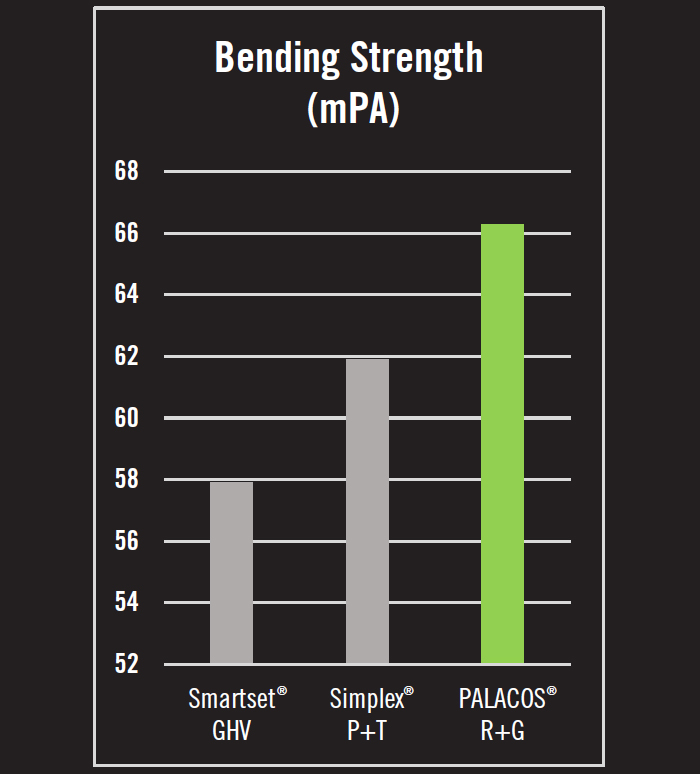
- Bending strength is the force required to deform or break a bone cement under stress. In tests of bending strength, PALACOS® R+G was 7.1% stronger than Simplex® P+T and 14.5% stronger than Smartset GHV (Figure 2).5
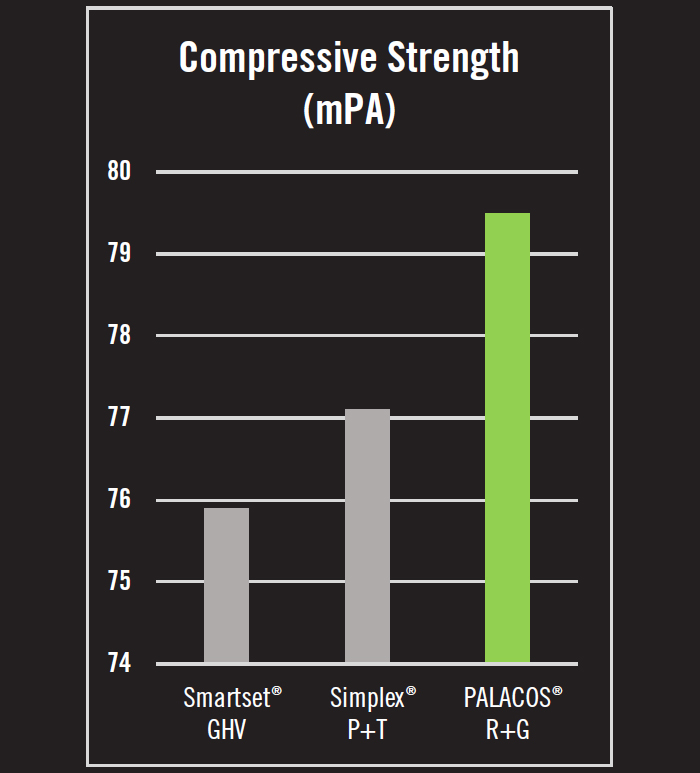
- Compressive strength is the maximum stress that a material can withstand before failure in compression. In compressive strength tests, PALACOS® R+G was 4.7% stronger than Simplex® P+T and 3.3% stronger than Smartset GHV (Figure 2).6
- Taking the average of these findings, PALACOS® R+G is 11.8% stronger than the other leading brands of antibiotic loaded bone cement in the US.
A more complete picture of how bone cements hold up in vivo may result from fatigue testing in which a strip of bone cement is subjected to sinusoidal bending and/or compression until it fails. In fatigue testing, investigators have identified a number of factors that appear to affect the durability of bone cement:
- One of the factors affecting the fatigue life of a bone cement is the co-polymer blend. The PALACOS® family of bone cements contain MA co-polymer which is more ductile than other copolymers (e.g. stryrene, pure PMMA base) and thus shows greater fatigue life and strength of the cement over cyclic testing than cements with these other copolymers.
- Another factor affecting fatigue life is the method used to sterilize the bone cement. Constantly innovating to maintain product excellence, Heraeus pioneered the use of ethylene oxide (EtO) sterilization for bone cements. While more costly, EtO preserves the molecular weight of PMMA cements and enables long chains to form during the polymerization process-providing significant strength and durability to the finished PALACOS® products.