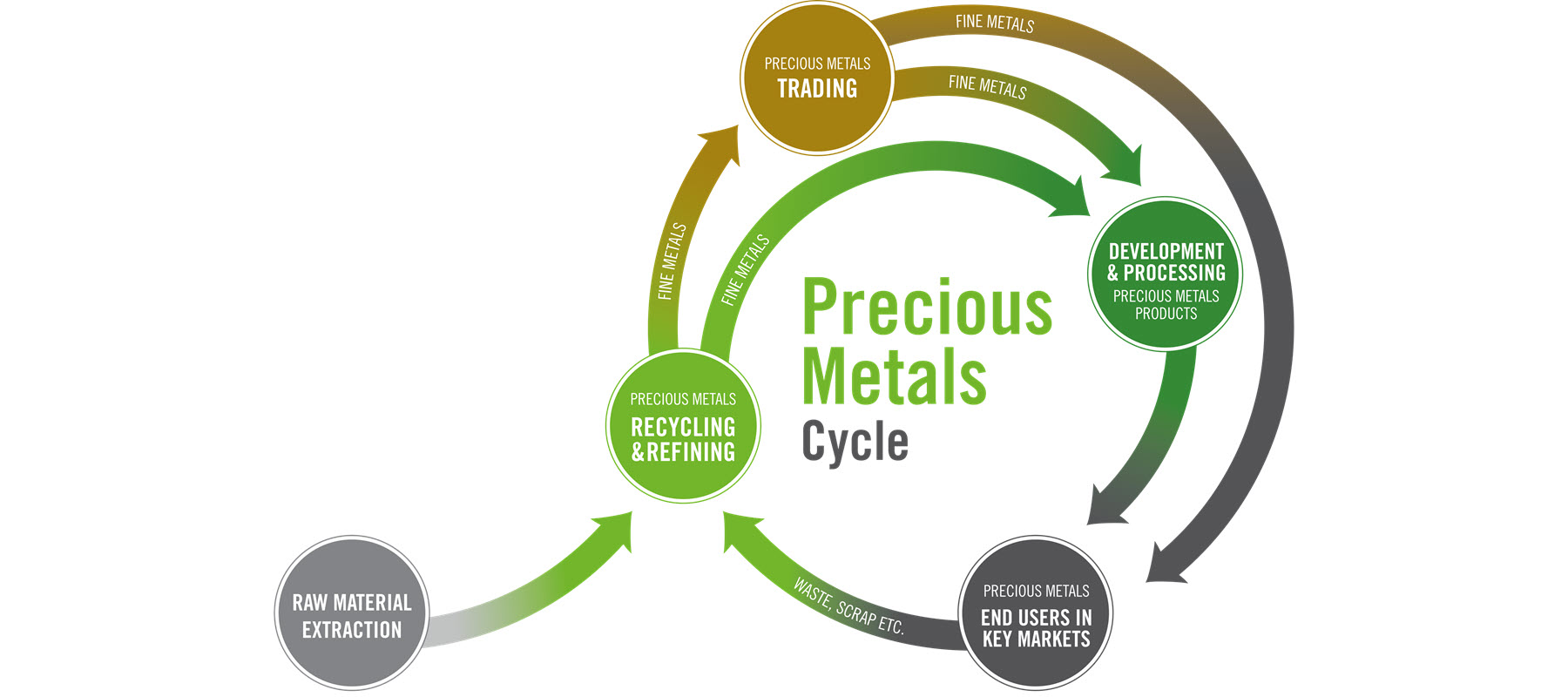
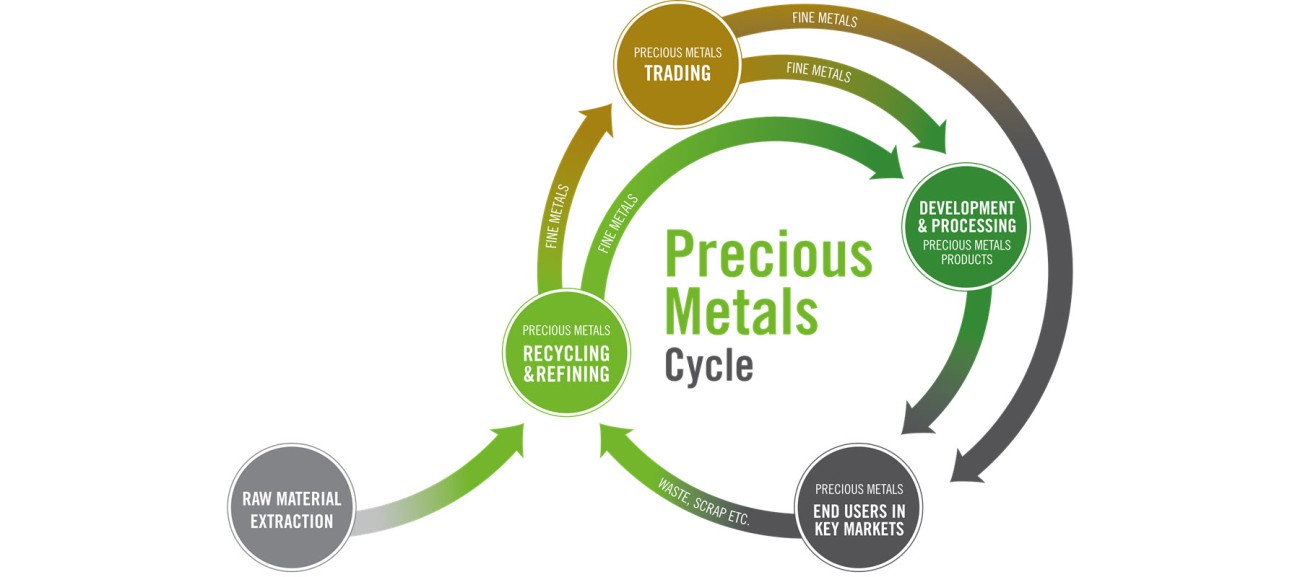
We are one of the world’s largest refiners of precious metals and a leading name in industrial precious metals trading. Our precious metals products are used in a wide variety of industries, including the automotive, chemicals, semiconductor, pharmaceutical, hydrogen and jewelry industry. We offer top quality solutions and products based on many years of experience and technical expertise. We are a reliable development partner for our customers and find the best solutions for their requirements.
With five trading and 14 production and recycling sites in all relevant time zones, we offer our customers a global production and logistics network. Trust and reliability, strict adherence to leading compliance standards, transparency and our financial stability have formed the foundation of our business for more than 170 years.